Ybco is a superconducting ceramic.
Ions within lattice structure ceramic.
Ceramics are a little more complex than metallic structures which is why metals were covered first.
This structure contains sulfide ions on the lattice points of an fcc lattice.
Its structure consists of three cubes with yttrium or barium at the centre copper at the corners and oxygen at the middle of each edge with the exception of the middle cube which has oxygen vacancies at the outer edges.
However the grid structure can take different forms which will be discussed in more detail in the article important types of lattice structures.
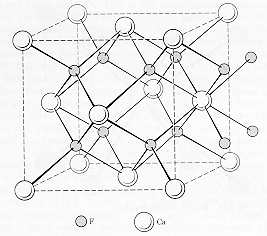
The coordination number of sodium is 6.
A ceramic has traditionally been defined as an inorganic.
If you examine the figure below you will see that there are six chloride ions immediately surrounding a single sodium ion.
The arrangement of sulfide ions is identical to the arrangement of chloride ions in sodium chloride the radius of a zinc ion is only about 40 of the radius of a sulfide ion so these small zn 2 ions are located in alternating tetrahedral holes that is in one half of the tetrahedral holes.
Larger anion radius most ionic crystals can be considered as close packed structure of anions.
As discussed in the introduction ceramics and related materials cover a wide range of objects.
Electrical charge crystal unit cell must remain electrically neutral sum of cation and anion charges in cell is 0 relative size of the ions ceramic crystal structure the ratio of ionic radii r cation r anion dictates the coordination number of anions around each cation.
Structure is determined by two characteristics.
The arrangement of sulfide ions is identical to the arrangement of chloride ions in sodium chloride the radius of a zinc ion is only about 40 of the radius of a sulfide ion so these small zn 2 ions are located in alternating tetrahedral holes that is in one half of the tetrahedral holes.
This structure contains sulfide ions on the lattice points of an fcc lattice.
3 ionic crystals cation radius nm anion radius nm 0 100 0 133 0 072 0 14 0 102 0 182 0 053 0 140 0 040 0 140 note.
The arrangement of sulfide ions is identical to the arrangement of chloride ions in sodium chloride the radius of a zinc ion is only about 40 of the radius of a sulfide ion so these small zn 2 ions are located in alternating tetrahedral holes that is in one half of the tetrahedral holes.
Acting forces on the metall ions a crystalline structure is a typical feature of metals.
The structural data on compounds which crystallize with the cubic pyrochlore structure have been analyzed to obtain a model equation that predicts their lattice constants.
The coordination number is the number of ions that immediately surround an ion of the opposite charge within a crystal lattice.